Model-informed dosing in pregnancy
'Virtual pregnancies'
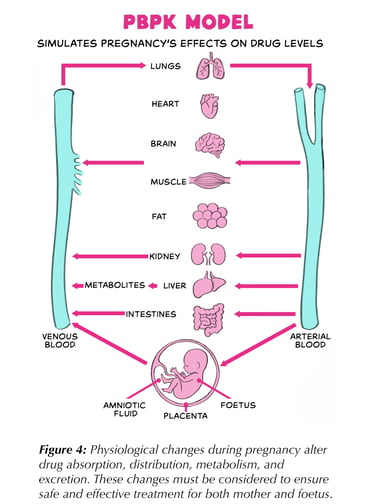
As mentioned earlier, traditional evidence on physiological changes during pregnancy is often limited. Physiologically-based pharmacokinetic (PBPK) models — essentially a computer-based “virtual body” — can help bridge this gap and support model-informed dosing recommendations. A brief outline of how PBPK model-informed dosing works is provided below.
Step 1: Create body
framework
The model is
divided into compartments representing major organs and tissues (Figure 4). Each compartment is assigned realistic values for size, blood flow, and other
physiological properties. For pregnancy models, these values are adjusted to reflect changes in each trimester, plus the placenta and foetus are added as extra compartments.
Step 2: Add drug-specific data
The model incorporates the drug’s physicochemical properties, such as solubility, protein binding, and how easily it moves between water and fat, along with laboratory and clinical data
on how it is absorbed, metabolized, and excreted.
Step 3: Link physiology & drug behaviour
Mathematical equations describe how the drug moves between compartments (via blood flow) and how it is eliminated (e.g., through liver metabolism or kidney
filtration).
Step 4: Test against real-world data
Before using it for pregnancy predictions, the model is checked against high-quality pharmacokinetic data from non-pregnant adults to ensure it accurately predicts real drug
concentrations over time. Once this “base model” works well, pregnancy-specific changes are added and tested against any available data from pregnant women.
Step 5: Generate
simulations
When verified, the model can run virtual clinical trials, predicting how
different doses will behave in a population of “virtual pregnant patients”, helping to select regimens that are both safe and effective for mother and baby.
