Model-informed dosing in pregnancy
The best dose
Project Madam issues evidence-based dose recommendations for pregnant women and their unborn children. Despite the widespread use of medication during pregnancy, pregnancy-specific clinical data regarding dosing are scarce. Resulting that most medicines prescribed in pregnancy are often based non-pregnant adult data, which in turn can cause uncertainty about efficacy, safety, and drug interactions. An important reason for this is the limited inclusion of these populations in clinical trials (for a variety of reasons including ethical, regulatory and logistical challenges), resulting in a lack of pharmacokinetic data and off-label drugs use.
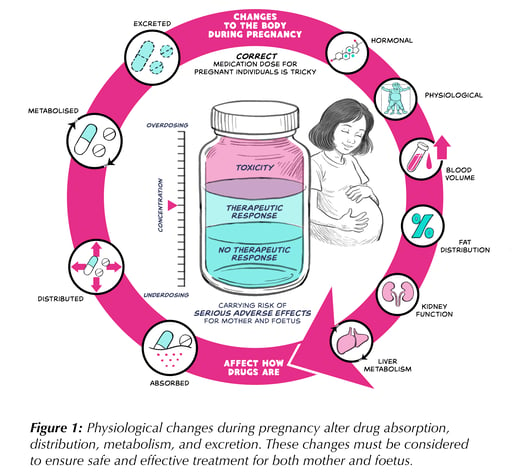
This lack of evidence leaves a gap in our understanding of how drugs behave in pregnant patients. During pregnancy, a woman’s body undergoes major physiological changes that can profoundly alter how drugs are absorbed, distributed, metabolized, and excreted. The effects vary by drug: increased plasma volume and body fat can dilute hydrophilic and lipophilic medications differently, liver enzyme activity may speed up or slow metabolism, and higher renal clearance can accelerate elimination of renally excreted drugs.
Placental transfer is another key consideration, determining how much drug reaches the foetus. Transfer depends on drug properties—molecular weight, lipophilicity, protein binding, and ionization—and the timing of exposure, which shapes whether foetal effects are therapeutic, developmental, or potentially harmful. Together, these factors guide whether a medicine should be used in pregnancy and whether maternal or foetal dosing adjustments are needed.
Thus, a dose that works in a non-pregnant adult may be ineffective—or unsafe—during pregnancy. Without adjustment, therapy risks under-treating the mother or exposing the foetus to excessive drug levels. Clinicians must consider altered pharmacokinetics, placental transfer, and gestational timing to ensure safe and effective treatment. Figure 1 illustrates the key factors supporting optimal dosing for both mother and child.
Next: Read about absorption, distribution, metabolism & excretion
