Sotalol
The full, clinically endorsed dose recommendation should be obtained from Lareb.
Rationale for drug selection
Sotalol is used during pregnancy for the treatment of foetal indications, including supraventricular tachycardia and atrial flutter. Foetal tachycardia lacks a standardized pharmacological treatment guideline. To support more evidence-based dosing during pregnancy, we assessed foetal efficacy targets, whether current maternal dosing achieves adequate foetal exposure.
Pharmacokinetics of sotalol in pregnancy
Sotalol is mainly eliminated by the kidneys. Due to increase of GFR during pregnancy, the drug concentrations in both the mother and the foetus decrease. Sotalol extensively crosses the placenta, with foetal plasma levels similar to maternal concentrations. To optimize dosing during pregnancy, maternal-foetal PBPK model was used to simulate the effects of different dosing strategies. The simulations indicated that the maximum daily dose can be safely reduced without compromising foetal efficacy, thus minimizing the risks associated with maternal overexposure.
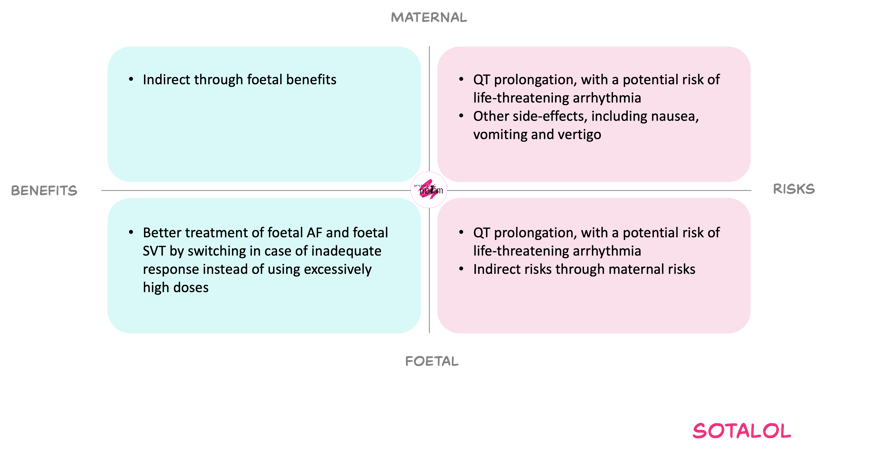
Benefits and risks with proposed dose adjustments
For the mother, the proposed decreased dose strategy aims to limit toxic exposure associated with QT prolongation and torsades des pointes, while still permitting adequate transplacental therapy. The incidence of common dose-related side effects such as nausea, vomiting and dizziness may decrease with proposed dose adjustments.
For the foetus, effective foetal exposure can restore sinus rhythm or control tachycardia, thereby reducing the risks of hydrops, neurological damage and intrauterine death. PBPK simulations indicate that, beyond a certain maternal dose, relatively few additional simulated foetuses achieve therapeutic concentrations while a substantial amount of simulated mother achieve toxic concentrations.
On this basis, and given the option to switch to alternative agents or combination therapy when response is inadequate at the capped dose, the Working Committee considered the proposed ceiling and dosing pattern an acceptable balance between achieving foetal efficacy and minimising maternal and foetal cardiac toxicity.
The expected benefits and associated risk of lowering the maximal daily dose during pregnancy.
In short
Sotalol is used to treat foetal arrythmias. During pregnancy, the mother clears sotalol faster, which can lower the amount that reaches the foetus. Yet doses high enough to push maternal concentrations above the QT-prolongation threshold may pose substantial risk without clear extra foetal benefit. PBPK model simulations indicated that the maximum daily dose can be safely reduced without compromising foetal efficacy, thus minimizing the risks associated with maternal overexposure. Based on the weighing of benefits and risks, the working committee derived an appropriate dose adjustment. Consult Lareb for the model-informed dosing recommendation.