Metformin
The full, clinically endorsed recommendation should be obtained from Lareb.
Rationale for drug selection
Metformin is used for gestational diabetes mellitus and (pre‑gestational) type 2 diabetes in pregnancy. It is known that pregnancy alters pharmacokinetics. Therefore, dose adjustments might be needed to maintain adequate glycaemic control. Diabetes in pregnancy carries substantial maternal and foetal risk, so appropriate dosing is clinically important.
Pharmacokinetics of metformin in pregnancy
During pregnancy, both renal blood flow and glomerular filtration are elevated, which leads to an increased renal clearance of metformin. This may result in lower plasma concentrations during pregnancy. As a result, pregnant patients may need a higher dose to maintain efficacy. Across studies, maternal metformin concentrations are decreased by about 20–35% during the third trimester of pregnancy. These alterations in plasma concentrations were also confirmed by maternal PBPK models, which provided strong evidence on the influence of gestation on the pharmacokinetics of metformin. These models were used for dosing and support decision making. Placental transfer of metformin occurs, and foetal concentrations can approximate maternal levels.
Benefits and risks with the proposed dose adjustments
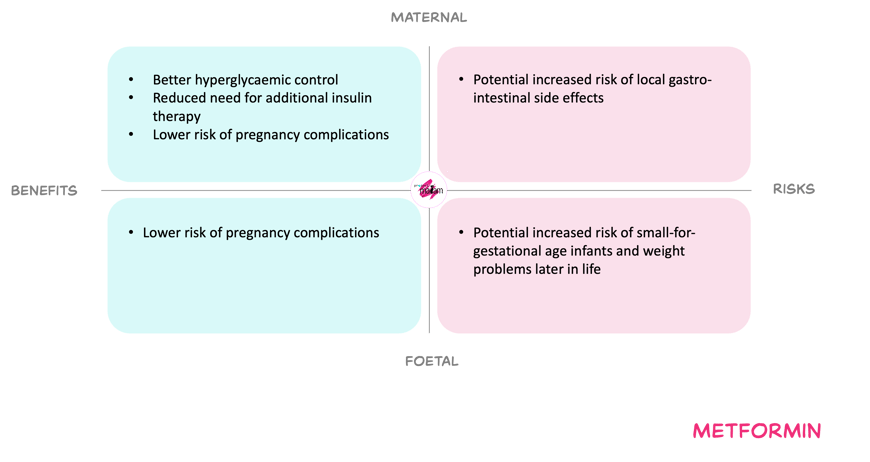
For the mother, increasing the dose to compensate for the fall in exposure in third trimester, may improve glycaemic control. Better control may reduce risks such as pre-eclampsia, stillbirth and other diabetes-related complications, and may reduce or delay the need for additional insulin when this is contra-indicated or not preferred. Gastro-intestinal adverse effects remain dose-limiting but are usually transient and manageable with gradual titration. Overall, no new maternal toxicity signals have been identified within the dose range already used in nonpregnant adults.
For the foetus, metformin crosses the placenta and foetal exposure is expected to be similar to maternal exposure. Available data do not suggest an increased risk of congenital anomalies or clear adverse perinatal outcomes. However, some studies suggest a possible increase in small-for-gestational-age infants and higher weight later in childhood, while others do not confirm this, so long-term effects remain uncertain and dose–response relationships are poorly defined.
The MADAM Working Committee balanced these uncertainties against the need for adequate diabetes control.
In short
Metformin is prescribed for gestational diabetes and diabetes type 2 during pregnancy. Renal clearance increases during pregnancy, which reduces metformin exposure, especially in third trimester. Clinical data and PBPK modelling together support the view that higher doses, similar to those used in nonpregnant adults, may be needed in some women to maintain glycaemic control. Based on the weighing of benefits and risks, the working committee derived an appropriate dose adjustment. Consult Lareb for the model-informed dosing recommendations.