Labetalol
The full, clinically endorsed recommendation should be obtained from Lareb.
Rationale for drug selection
Labetalol is used to manage hypertension in pregnancy. Pregnancy can alter the pharmacokinetics of drugs. Therefore, dose adjustments might be needed to sustain blood pressure control while minimising maternal and foetal risks.
Pharmacokinetics of labetalol in pregnancy
Pregnancy alters the activity drug-metabolising enzymes and thereby elimination of labetalol. Specifically, CYP2C19 activity is reduced, while UGT1A1 and UGT2B7 activities are increased. The renal clearance of labetalol is also enhanced during pregnancy. These changes taken together lead to lower plasma concentrations and faster overall clearance during the second and third trimester of pregnancy. The alterations in plasma concentrations have been confirmed by a population-pharmacokinetic model. The model provided strong evidence on the influence of gestational age on the pharmacokinetics changes of labetalol in pregnancy, informing alternative dosing strategies and supporting decision-making. PBPK models have been explored but were not considered sufficiently robust for dose simulations.
As a result, pregnant patients may need both a higher dose and a more frequent dosing schedule to maintain stable labetalol levels. Labetalol crosses the placenta, reaching cord concentrations that are typically about 50-60% of maternal levels.
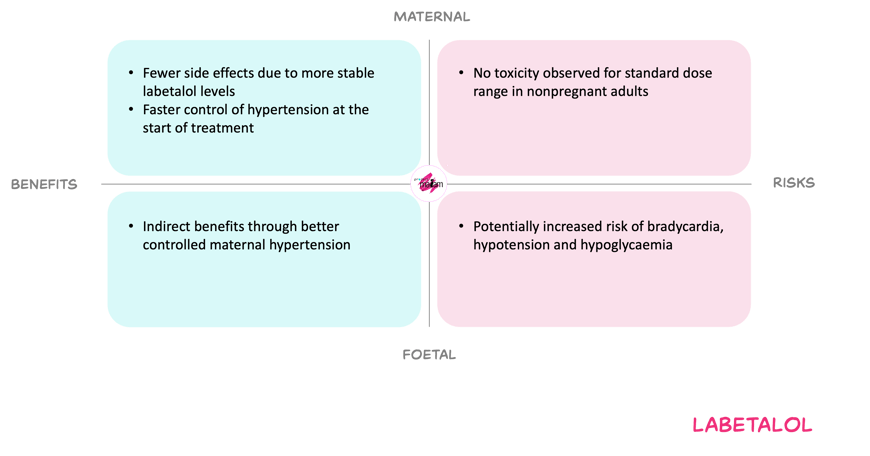
Benefits and risks with the proposed dose adjustments
For the mother, using a higher dose frequency aims to counter increased clearance and the short half-life, thereby improving early blood pressure control and avoiding large peak–trough fluctuations. This may reduce symptoms such as dizziness from rapid blood pressure swings and may lessen the need for very high total daily doses later in pregnancy. The main maternal risks remain dose-related beta-blocker effects such as hypotension and bradycardia, but these are limited when treatment is titrated against blood pressure and remain within standard adult dose range.
For the foetus, better controlled maternal hypertension is expected to reduce the risk of placental insufficiency, growth restriction and other hypertensive complications. Labetalol crosses the placenta and later-pregnancy beta-blocker exposure may be associated with neonatal hypoglycaemia, bradycardia, hypotension and possible small-for-gestational-age birth. Higher maternal doses could theoretically increase these neonatal effects, but dose relationship has not been demonstrated. The MADAM Working Committee therefore considered increasing dose and frequence while remaining in the standard dose range, an acceptable balance of benefits and risks.
In short
Labetalol is prescribed for hypertension during pregnancy. Maternal plasma concentrations of labetalol are reduced during pregnancy, especially in the second and third trimesters. Consequently, some patients may require a higher starting dose and more frequent dosing compared to non-pregnant individuals to maintain effective blood pressure control and more stable levels, supporting both maternal and foetal outcomes. Consult Lareb for the model-informed dosing recommendations.