Citalopram
The full, clinically endorsed recommendation should be obtained from Lareb.
Rationale for drug selection
Citalopram is a selective serotonin reuptake inhibitor (SSRI) used in pregnancy to treat depressive, panic, and anxiety disorders. Because pregnancy alters the activity of drug metabolizing enzymes, dose-adjustments may be necessary to maintain effective concentrations during pregnancy.
Pharmacokinetics of citalopram in pregnancy
Citalopram is mainly metabolized by hepatic CYP enzymes such as CYP2C19, CYP3A4 and CYP2D6. While the activity of CYP2C19 decreases during pregnancy, the activity of CYP3A4 and CYP2D6 is significantly elevated. Pharmacokinetic studies confirm that citalopram concentrations can decrease by 40% during pregnancy, particularly in the second and third trimester. As a result, some pregnant women may benefit from an increased dosage when a reduced effect of citalopram is observed. Citalopram crosses the placenta, with foetal levels that are approximately 60–80% of maternal concentrations.
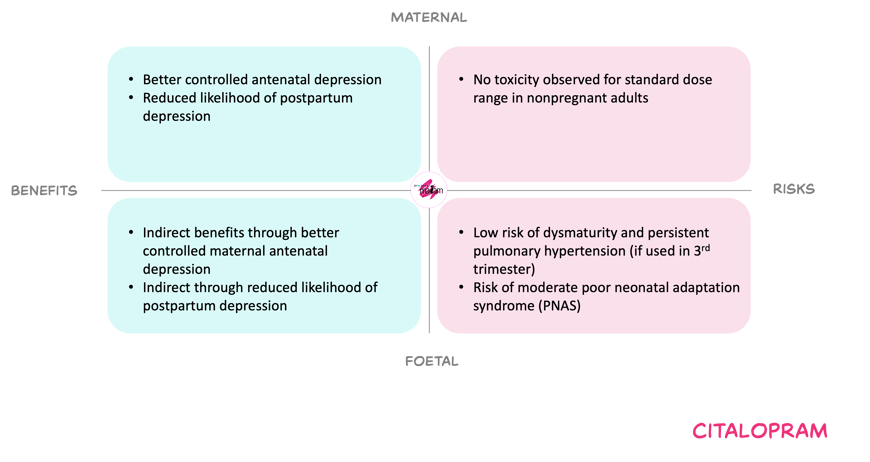
Benefits and risks with the proposed dose adjustments
For the mother, an option to increase the dose may reduce the risk of relapse or persistent depressive and anxiety symptoms, and may also lower the risk of postpartum depression. Side effects and dose-related toxicities (such as QT prolongation, serotonin syndrome or seizures) appear uncommon within the usual dose range, and pregnancy itself seems not to amplify these risks.
For the foetus, citalopram crosses the placenta and long-term experience does not suggest a major increase in overall congenital anomaly risk. However, late-pregnancy exposure is associated with poor neonatal adaptation syndrome, which is generally mild and transient. Also, a low incidence of persistent pulmonary hypertension of the newborn has been reported, which could not been confirmed by other studies. Untreated or undertreated maternal depression and anxiety carry recognised risks, including harmful behaviours, prematurity and growth restriction. Therefore, maintaining effective maternal treatment may indirectly benefit the foetus. Balancing these factors, the Working Committee considered the residual neonatal risks acceptable in view of the maternal and indirect foetal benefits, provided that dosing stays within recommended limits.
In short
Pregnancy may reduce maternal plasma concentrations throughout pregnancy, especially later gestation, with possible symptom deterioration. Neonatal adaptation problems and possible PPHN remain important but generally infrequent concerns, weighed against the risks of inadequately treated maternal disease. Dose adjustments may therefore be needed to maintain effective treatment, but the exact regimen should be guided by clinical response. Consult Lareb for the dosing recommendations.